Bard PowerPort Lawsuit
Hundreds of adverse events associated with Bard PowerPorts have been reported to the FDA, and lawsuits are being filed. If you have been injured after being implanted with a Bard PowerPort device, you may have a claim. Contact The Lanier Law Firm at 800-723-3216 today for a free case evaluation.
Home » Bard PowerPort Lawsuit

Legally Reviewed By: Rebecca Phillips
Mass Torts Director
Co-Lead Plaintiffs Counsel – Bard PowerPort Multi-District Litigation
- Page Last Updated:
- July 7, 2025

Legally Reviewed By: Rebecca Phillips
Mass Torts Director
Co-Lead Plaintiffs Counsel –
Bard PowerPort Multi-District Litigation
- Page Last Updated:
- July 7, 2025
Why Choose the Lanier Law Firm to Handle Your Bard PowerPort Lawsuit
What Is the Problem with Bard PowerPorts?
The Effects and Complications Associated with Bard Powerport Catheters
Liability for Defective PowerPort Catheter Defects
When Should I Contact a Bard PowerPort Attorney?
“Hi, my name’s Rebecca Phillips and I’m an attorney at The Lanier Law Firm, where we’re helping individuals who have been harmed by Bard PowerPorts. In fact, we’ve recently been named as a co-lead in the Bard PowerPort Multidistrict Litigation. So, what is a Bard PowerPort? Well, it’s a medical device that is implanted into a patient’s body when a doctor will need repeated access to the patient’s vascular system. For example, the Ports can be used to administer IV nutrition, or they can be used to administer medicine, like chemotherapies, or they can be used to withdraw blood when the patient’s going to need blood withdrawn on a regular basis.
So what’s wrong with these Ports? Well, the lawsuits allege that these devices are defective, and they can fail in one of three ways. Number one, they can fracture, and when they fracture, pieces of the device can end up in some pretty scary places, like a patient’s heart. Number two, they can cause severe life-threatening infections. And number three, they can cause blood clots. And these blood clots can also be life-threatening.
So what’s the defect? Well, the lawsuits allege that barium sulfate was left exposed on the outer surface of these devices, and when the devices were implanted, barium sulfate degrades in vivo, which causes divots and cracks on the surface of the device. It creates the places where the fracture points happen, where the breaks happen, and it creates divots that bacteria can hide in and increase the risk for blood clots.
That’s why The Lanier Law Firm is pursuing this litigation on behalf of clients who have been injured by these devices. So if you or someone you know has had a Bard PowerPort implanted in their body and has experienced one of these injuries, please give us a call, and we’ll do a free assessment on whether or not you might have a case. We’re here to help. Again, my name’s Rebecca, and I work at The Lanier Law Firm, where we are in the co-leadership of the Bard PowerPort Multidistrict Litigation. We’re happy to help.” – Rebecca Phillips, Mass Torts Director
At the Lanier Law Firm, we are passionate about holding large corporations accountable for harming individuals. When you hire the Lanier Law Firm, you can trust that our passionate and experienced pharmaceutical liability lawyers will aggressively seek justice.
Bard PowerPorts can cause serious life-threatening complications, including blood clots, infections, and vital organ damage, according to lawsuits filed nationwide against manufacturer C.R. Bard, a wholly-owned subsidiary of Becton Dickinson and Co.
The U.S. Food and Drug Administration has received hundreds of adverse event reports from patients implanted with Bard PowerPorts, but the company continues to market the products as safe and effective without recalling them, adjusting the product, or adding warnings to the label.
By submitting this form, you agree to our terms & conditions. Please read full disclaimer here.
Bard PowerPort Case Timeline
May 14, 2025 – The Court has selected six bellwether cases for trial in 2026. The Court also selected one alternate case, should one of the other six cases be resolved before trial. Of the seven cases, three are infection injuries, two are thrombus/occlusion injuries, and two, including the alternate, are fracture injuries. This selection will be followed by a short period of additional discovery and submission of case-specific expert reports, and briefing on summary judgment and expert challenges begins in the fall.
May 7, 2025 – General and case-specific discovery for Bellwether Group 1 has closed, and the parties will submit briefing today about which six cases should move forward as bellwethers. After the Court decides that issue, additional case-specific discovery may continue. The parties are also in the process of exchanging expert reports, rebuttals, and beginning expert depositions.
April 9, 2025 – Plaintiffs served their general expert reports on March 14, 2025. Plaintiffs disclosed a dozen experts who support Plaintiffs’ claims that Defendants’ port catheters are defective: Michael Beatrice, PhD; Bernard Camins, MD; Ahmed El-Ghannam, PhD; Darren Hurst, MD; William Jarvis, MD, Madris Kinard, MBA; Deborah Leckband PhD; Brian McVerry, PhD; Buddy Ratner, PhD; Amir Sheikhi, PhD; Becky Smith, MD; and Jeffrey Weinstein, MD.
The parties have completed general discovery and are winding up case-specific discovery for bellwether selection. At the last status conference, per Defendants’ request, the Court moved the deadline for the parties to agree on six bellwether cases or to submit opposing briefing about representative cases to April 28. The next conference is set for May 1.
January 16, 2025 – Discovery continues, and depositions are ongoing. This month, depositions of 15 potential bellwether plaintiffs will begin, followed soon after by treating physicians. These depositions are intended to help the parties narrow the bellwether pool from 15 cases down to 6 cases that will be tried in early 2026.
In December, the parties could not reach agreement over topics for corporate representative deposition, and the Court issued an order to govern the issue. The next hearing is set for today, January 16, 2025, and the parties are expected to argue over Defendants’ withholding of non-privileged evidence. Plaintiffs have already been successful in getting Defendants to release such evidence through the course of earlier disputes.
October 17, 2024 – The Court held a hearing on October 3 to discuss the progress of discovery. The parties argued regarding Defendants’ claimed privileges and redactions of evidence and regarding a potential, short extension of the discovery schedule due to Defendants late production of documents and continual moving back of witness deposition dates. The Court ordered more briefing on the issues. The parties also argued about evidence regarding the materials out of which Defendants’ catheters are made, and the Court determined that, if in the future Plaintiffs discovery that Defendants have withheld information, they may re-approach the Court regarding an appropriate sanction.
Meanwhile, depositions continue. Co-lead counsel, LLF attorney Rebecca Phillips, said that, while witness testimony cannot yet be made public, “Our team is doing good work in there.”
September 16, 2024 – Discovery continues, and depositions of Defendants’ witnesses have begun. The Court held a conference with the parties on August 16, 2024, to discuss the status of the case and ongoing discovery issues. The biggest issue was that, from the start of discovery, Defendants have opposed Plaintiffs’ discovery into the corporate relationship between Bard and BD for the purpose of proving successor liability. The Court – for a second time – rejected the Defendants argument. Unless the parties are able to reach an agreed stipulation on the relationship between Bard and BD, Plaintiffs’ successor liability discovery will continue.
Another hearing is scheduled for October, and at the last status conference, our own Rebecca Phillips who is on the leadership team for this litigation, told the Court that it may anticipate an argument about evidence that Defendants are withholding as privileged.
June 11, 2024 – The Court held another status conference with the parties on May 24, 2024 to receive an update on continued discovery issues. At present, the parties remain on track with the production of evidence so that depositions can begin in August of 2024. According to co-lead counsel, Rebecca Phillips, an attorney at the Lanier Law Firm, “It is important to get these cases to trial as quickly as we reasonably can. Since many of our clients are very ill, they can’t see justice soon enough.”
May 7, 2024 – The parties are scheduled to appear before the MDL Court again on May 10 to discuss ongoing discovery issues, including whether the parties are on track to meet current discovery deadlines. At present, the parties continue to engage in the discovery process of identifying relevant witnesses and documents for production. Depositions of those witnesses are still scheduled to begin in August of this year.
April 22, 2024 – The bellwether pool closed on April 1, with bellwethers to be selected in the coming months. The parties continue to work through general discovery issues, and depositions are set to begin in August of this year.
March 8, 2024 – The Court held a case management conference on March 1, 2024, during which the Defendants argued to severely restrict the discovery that the Plaintiffs may conduct. The Defendants were unsuccessful. The parties will meet with the Court again on March 29, 2024, to discuss the progress of discovery.
February 2. 2024 – Discovery is ongoing, and the parties are due back in court in Phoenix on March 1, 2024. At that time, the parties will report their progress in discovery and raise any discovery disagreements with Judge Campbell. The judge has also asked that the parties finalize a preservation order that would apply to port devices that are explanted because of injury.
November 16, 2023 – Judge Campbell held a status conference at his courtroom in Phoenix, Arizona. After the conference, he entered a Master Complaint and a Short-Form Complaint so that Plaintiffs may file their case directly into the MDL. Judge Campbell also entered a case management order governing case deadlines. The discovery period during which Plaintiffs may gather their evidence will run from November 20, 2023, through January 31, 2025. After that time, the parties will submit expert reports, and trials are anticipated to begin in 2026.
October 18, 2023 – Judge Campbell has ordered the parties to cooperate in filing proposed protective orders, electronic discovery orders, and preservation orders by October 27, 2023. He also ordered the parties to meet and confer regarding the form of complaints and direct filing orders, among other preliminary issues. According to attorney Rebecca Phillips, “We will work with the Defendants’ counsel to help the Court get the MDL up and running as efficiently as possible as soon as possible.” The next hearing will occur in Phoenix on November 16, 2023.
September 19, 2023 – The US District Court for the District of Arizona has appointed Lanier Law Firm’s own Rebecca Phillips as Co-Lead Counsel on the Plaintiff’s Leadership Counsel on MDL No. 3081, In Re: Bard Implanted Catheter Product Liability Litigation.
August 9, 2023 – Yesterday, the Judicial Panel on Multidistrict Litigation centralized federal PowerPort cases in the United States District Court for the District of Arizona before the Honorable David Campbell. Judge Campbell was appointed to the bench in 2003 and, since that time, has presided over multiple MDLs, including In re: Bard IVC Filters Products Liability Litigation, case number 2:15-md-02641.
July 27, 2023 – Plaintiffs argued before the Judicial Panel on Multidistrict Litigation (“JPML”) that PowerPort cases should be consolidated for pretrial proceedings. Plaintiffs proposed consolidation in the Western District of Missouri, a place where cases have already been filed. Defendant opposed consolidation on the basis that some Plaintiffs injuries were, in its view, not attributable to the PowerPort. Defendant proposed either Utah or Arizona as appropriate venues if the JPML was inclined to consolidate the cases.
June 16, 2023 – The United States Judicial Panel on Multidistrict Litigation filed a notice that it will hear oral arguments on July 27, 2023, in San Francisco about whether to create a centralized federal MDL. If created, all pending litigation and any future lawsuits filed in federal court will be transferred to a single judge regardless of where they originated. The judge is likely to institute a selection process to hold trials in a few bellwether cases.
May 24, 2023 – Plaintiffs in various cases requested that the United States Judicial Panel on Multidistrict Litigation consolidate Bard PowerPort Lawsuits into a multidistrict litigation and that the cases be transferred to the Western District of Missouri. According to the motion, at least ten lawsuits have been filed in various federal districts nationwide, and many more are expected.
July 8, 2019 – The FDA cleared Bard Power-Injectable Implantable PowerPorts through the 510(k) process.
June 7, 2019 – The FDA makes the ASR database public. In response to the public outcry to the Kaiser Health report, and starts requiring companies to report adverse events through the publicly accessible MAUDE database.
March 7, 2019 – Kaiser Health publishes a report revealing that medical device companies were filing reports of device malfunctions to the FDA in secret.
December 29, 2017 – Becton Dickinson and Co. acquires C.R. Bard and is believed to have taken on Bard’s liabilities. This company earns approximately $16 billion in annualized revenue.
February 14, 2014 – The FDA clears the PowerPort Implantable Port through the 510(k) process.
November 15, 2012 – The FDA clears the PowerPort Clearvue Slim Implantable Port through the 510(k) process.
March 27, 2009 – C.R. Bard receives a 510(k) clearance for the PowerPort Due MRI Implanted Port with 9.5 Fr. Dual Lumen Chronoflex Polyurethane Catheter.
June 4, 2008 – The FDA clears the Powerport Implanted Port with Groshong Catheter.
December 19, 2007 – C.R. Bard receives a 510(k) clearance for the MRI Powerport Implanted Port with 9.6 Fr. Silicone Catheter.
November 14, 2007 – C.R. Bard receives clearance through the FDA’s 510(k) process for the Titanium PowerPort Isp Implanted Port with 6 Fr Chronoflex Polyurethane Catheter.
November 1, 2007 – The FDA clears C.R. Bard’s Powerport Polymetric Port with 8 Fr S/L Chronoflex Catheter through the 510(k) process.
January 25, 2007 – C.R. Bard receives an FDA 510(k) clearance for the Powerport Polymetric Port with 8 Fr S/L Chronoflex Catheter.
July 14, 2006 – The FDA grants multiple 510(k) clearances for C.R. Bard’s Implanted Titanium Port with 8 Fr. Chronoflex Catheter.
Why Choose the Lanier Law Firm to Handle Your Bard PowerPort Lawsuit
Since our establishment in 1990, our nationally recognized defective products lawyers have won nearly $20 billion in verdicts and settlements on behalf of our deserving clients, including the following:
- $9 billion verdict against Takeda & Eli Lilly for cancer associated with the diabetes drug Actos
- $4.85 billion National Vioxx Settlement against Merck & Co.
- $4.69 billion verdict against Johnson & Johnson in the first trial linking asbestos in baby powder to ovarian cancer
- $1.05 billion against DePuy Orthopaedics and Johnson & Johnson for defective hip implants
- $253 million in the first verdict against Vioxx in the United States
- $57 million national settlement for Yaz birth control victims
- $56 million verdict against Biomet for defective metal-on-metal hip implants
Going against large drug companies like Becton Dickinson and Bard requires extensive financial resources that can be invested upfront. It also requires trial skills that can match the skills of the most expensive lawyers hired by the pharmaceutical companies.

At the Lanier Law Firm, we are not afraid to go against large companies. Law360 designated us the Most Feared Plaintiffs Firm in 2014. Our attorneys have also received prestigious awards, including the following:
- Best Lawyers in America
- Most Impressive Plaintiff Verdict by Courtroom View Network
- Best Law Firms, Tier 1 Ranking
- Largest Verdict of 2018, by Verdict Search and the National Law Journal
Our founder, Mark Lanier, has been recognized by the National Trial Lawyers as the 2018 Trial Lawyer of the Year and is included among their top 50 most influential lawyers in the United States.
What Is a Bard Powerport?
Bard Powerport is a brand name for a medical device designed for surgical implantation in the human body. It provides health care providers with direct access to a vein to deliver medications, intravenous fluids, nutrition, or blood products. It is a convenient method of avoiding frequent patient contact with needles. They are commonly used for chemotherapy in cancer patients.
What Is the Problem with Bard PowerPorts?
The catheter is made from a type of polyurethane and silicone. It contains barium sulfate, a contrast dye that makes the port more visible during imaging tests, such as X-rays and CT scans. Unfortunately, barium sulfate has been shown to degrade polyurethane and silicone when in contact with human tissue.
The Effects and Complications Associated with Bard Powerport Catheters
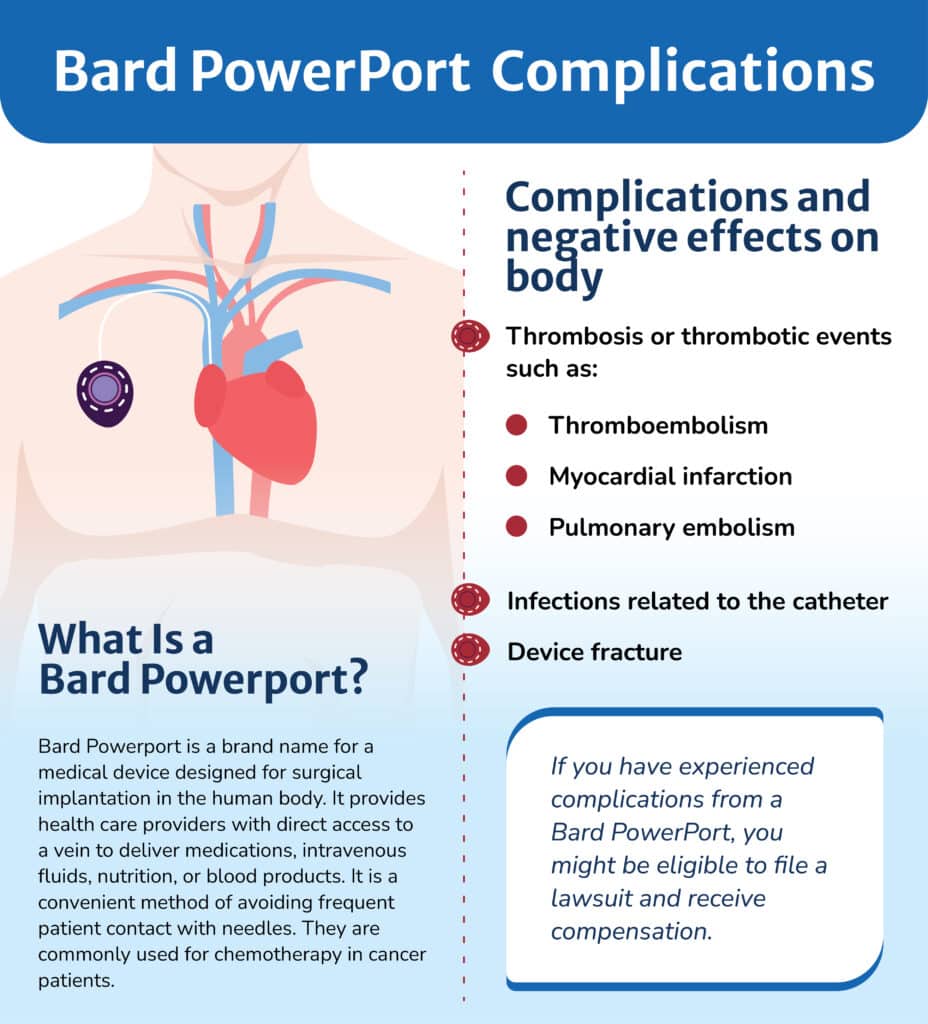
The complaints also allege that catheter particles enter the bloodstream and travel to various internal organs, causing organ damage and life-threatening infections. According to complaints, patients with the implant have an increased risk of the following:
- Thrombosis or thrombotic events such as:
– Thromboembolism – A life-threatening blood clot that blocks blood flow in the veins
– Myocardial infarction symptoms – Heart attack symptoms, such as chest pain, dizziness, and difficulty breathing
– Pulmonary embolism – a blood clot that has traveled to the lungs, which blocks blood flow to the lungs and can be deadly without emergency treatment - Infections related to the catheter
- Device fracture
Additionally, some plaintiffs also allege that they have experienced severe or persistent pain, tearing of internal body structures, hematomas, cardiac arrhythmia, and fluid buildup around the heart. Some have required surgery.
Complaints allege that the particles of barium sulfate break down from the surface of the PowerPort over time, leaving divots, pits, cracks, microfractures, and breakage of the device. The device may malfunction or migrate to other parts of the body.
Liability for Defective PowerPort Catheter Defects
Manufacturers of medical devices are required to ensure that the products they make available to the public are safe and effective.

What Did Bard Know About the PowerPort’s Defects?
According to the pending lawsuits, Bard received a large number of adverse event reports from health care providers soon after introducing the PowerPort to the market, indicating that the company knew or should have known that patients implanted with its PowerPorts had increased risks of potentially life-threatening injuries.
Who Is Liable for Harm Caused by Bard PowerPort Catheters?
Becton Dickinson acquired Bard in 2017 as a wholly-owned subsidiary. As a result, Becton Dickinson and Bard may be liable.
Complaint allege that when C.R. Bard began receiving adverse event reports, it may have prevented hundreds of injuries and deaths by doing even one of the following:
- Recall the product
- Remove the barium sulfate from the product
- Encapsulate the barium sulfate
- Use a different polymer formulation
- Update the warning label to disclose the known risks
Instead, Bard continues to market the product as safe and effective.
Complaints allege that not only did the company fail to take steps to protect the public, but it may have deliberately concealed the product’s harmful effects from the public.

When Should I Contact a Bard PowerPort Attorney?
Every state sets its own deadline for filing a lawsuit, known as the statute of limitations. If you do not file before the deadline expires or if your case is not tolled, you can lose your right to pursue justice for your injuries. For this reason, it is imperative that you contact an attorney as soon as possible after you discover your injuries.
Our hard-working and dedicated product liability attorneys can determine how the statute of limitations affects your case and ensure your case is filed on time.
Contact the Lanier Law Firm to Start Your Claim Today
If you or your loved one received any of the following Bard PowerPorts and suffered an injury due to catheter migration or fracture, including infections, organ damage, and life-threatening blood clots, you may have a case against Bard and Becton Dickinson:
- Bard Power-Injectable Implantable Ports (Powerports®)
- Bardport®, Slimport®, And X-Port® Implanted Ports
- Power-Injectable Implantable Ports With Chronoflex Polyurethane Catheters
- Powerport Implantable Port
- Powerport Clearvue Slim Implantable Port
- Powerport Duo MRI Implanted Port With 9.5 Fr. Dual Lumen Chronoflex Polyurethane Catheter
- Powerport Implanted Port With Groshong Catheter
- MRI Powerport Implanted Port With 9.6 Fr Silicone Catheter
- Titanium Powerport Isp Implanted Port With 6 Fr Chronoflex Polyurethane Catheter
- Titanium Powerport Isp Implanted Port
We have a winning record against drug companies and medical device manufacturers. Contact us today for a free case evaluation.
Frequently Asked Questions
Below are answers to common questions about Bard PowerPort lawsuits.
How Were Such Dangerous Products Cleared by the FDA?
Bard PowerPorts were cleared through the FDA’s Premarket Notification 510(k) program, which allows manufacturers to receive clearance for products that are, in theory, substantially similar to existing products without having to complete clinical studies verifying a product’s safety and efficacy.
Is the Bard PowerPort Device Recalled?
There is currently no active recall for these products. Some Bard PowerPorts were recalled in October 2019 for unrelated reasons, but this recall was terminated February 18, 2022.
Is There a Class-Action Bard PowerPort Lawsuit?
There is no class-action lawsuit, but a motion to consolidate the cases into multidistrict litigation, or MDL, has been filed.
When cases are transferred to an MDL, case discovery is shared, but each case remains separate. A few representative cases are usually selected to go to trial as a test to determine the likely outcome for the other cases. These are known as bellwether trials. These trials can provide guidance for how the remaining cases should resolve. Our mass tort lawyers have extensive experience with these types of cases and have served in leadership roles in large MDLs.
How Much Does a PowerPort Attorney Cost?
At The Lanier Law Firm, the initial consultation is free, and we work on a contingency fee arrangement. This means that we take on all the financial risks associated with your case, investing our own resources into filing fees, expert witnesses, and other case costs, with nothing due from you until after we recover compensation for you.
By submitting this form, you agree to our terms & conditions. Please read full disclaimer here.



